Exploring Ionic And Covalent Bonds Gizmo | Exploring ionic and covalent bonds gizmo : The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between atoms in a covalent bond. By doing this, atoms form bonds. Ionic and covalent bonds are the two extremes of bonding. Ionic bonds student exploration gizmo answer key read pdf ionic bonds student exploration gizmo answer key before using the gizmo.
Document content and description below. As you will see in the covalent bonds gizmo™, atoms form bonds in this way. Ionic and covalent bonds hold molecules together. Combinations of metals and nonmetals typically form ionic bonds. Describe the energetics of covalent and ionic bond formation and breakage.

What are ionic and covalent bonds? The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between atoms in a covalent bond. By the law of conservation of energy, when a new chemical bond is formed. The variety of different substances is a result of combining different elements, in different ratios, using different types of chemical bonds. The ionic bonds gizmo™ allows you to explore how ionic bonds form. Covalent bonding is dominant in organic chemistry, but ionic bonds generally have higher dissociation energies. By doing this, atoms form bonds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more complexity in life. In the second part of this lab you will explore the differences in melting points between ionically bonded and covalently bonded compounds. Ionic and covalent bonds hold molecules together. More soluble in water because of the strong positive and negative charge. Covalent bonds and ionic bonds are two different ways of how elements bond to each other.
Most metals can conduct and ionic has metal its bond. Ionic bond strength and lattice energy. Learn about ionic and covalent bond topic of chemistry in details explained by subject experts on vedantu.com. Covalent bonding is dominant in organic chemistry, but ionic bonds generally have higher dissociation energies. Register free for online tutoring session to the above discussion must have cleared your concepts related to ionic bond and covalent bond and why the former is partially covalent.
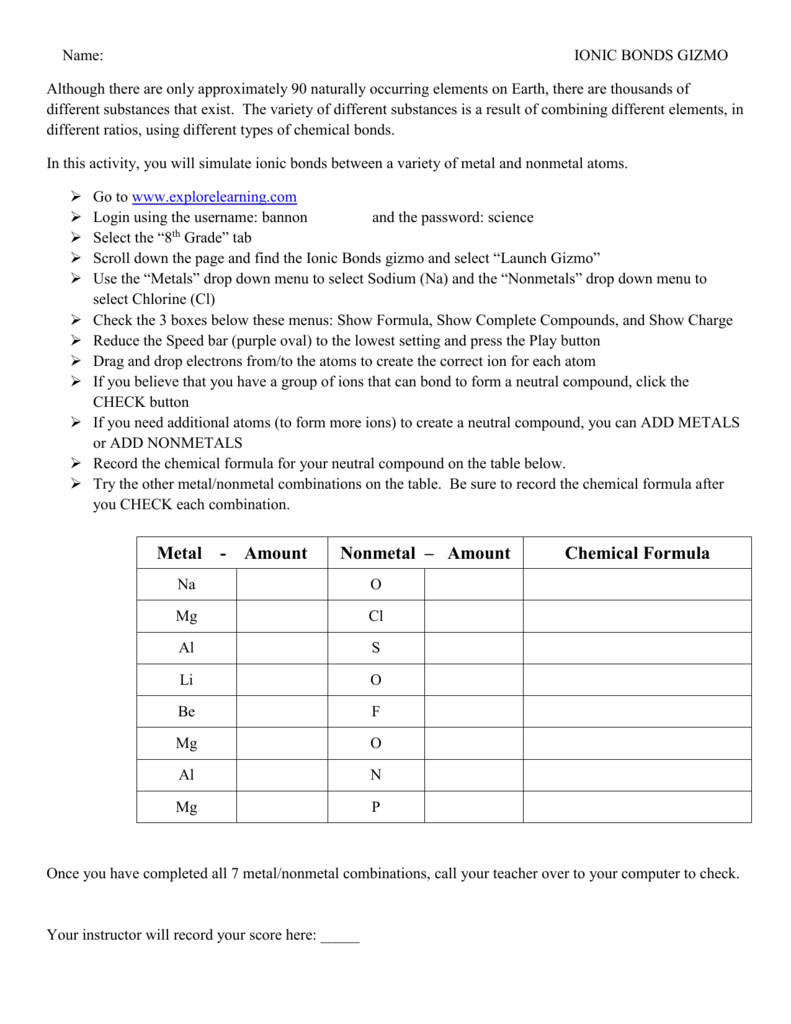
Select a metal and a nonmetal atom, and transfer electrons from one to the other. Register free for online tutoring session to the above discussion must have cleared your concepts related to ionic bond and covalent bond and why the former is partially covalent. Number bonds are a great way for your kids to explore the relationship between addition and subtraction. You can calculate the charge of an atom by subtracting the. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when covalent bonds have a definite and predictable shape and have low melting and boiling points. Learn vocabulary, terms and more with flashcards, games and other study tools. To begin, check that sodium (na) and chlorine (cl) are selected from the menus at form a bond: The ionic bonds gizmo™ allows you to explore how. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. To begin, check that fluorine is selected. Start studying covalent bonds gizmo. The ionic bonds gizmo™ allows you to explore how ionic bonds form. Describe the energetics of covalent and ionic bond formation and breakage.
You can calculate the charge of an atom by subtracting the. Ionic and covalent bonds are the two main types of chemical bonding. As mentioned above, ionic bonds are a result of electrostatic forces between atoms that get attracted towards each other due to the possession of opposite electrical charges. By doing this, atoms form bonds. The ionic bonds gizmo™ allows you to explore how.
Certainly more common in biology, but being more common. Chemical bond where electron(s) are shared between two nonmetals, giving the atoms involved a full octet. The ionic bonds gizmo™ allows you to explore how ionic bonds form. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between atoms in a covalent bond. By the law of conservation of energy, when a new chemical bond is formed. Ionic bonding is electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. Select a metal and a nonmetal atom, and transfer electrons from one to the other. As you will see in the covalent bonds gizmo™, atoms form bonds in this way. Just like students sharing markers, atoms sometimes share or swap electrons. To begin, check that sodium (na) and chlorine (cl) are selected from the menus at form a bond: Ionic and covalent bonding assessment: There are eight markers in a full. The ionic bonds gizmo™ allows you to explore how.
Exploring Ionic And Covalent Bonds Gizmo: Wont need as much heat to melt.
Post a Comment